Writers: Konstantin Tumanov, Ignacio Louzan
After the rush of R&D resources to pandemic-related treatments, the majority of funding has now swung back to oncology. This is more than timely as new cancer diagnoses in the U.S. are expected to hit a record number—over 2 million—in 2024, according to the American Cancer Society. Personalized immunotherapies are where expectations are highest. As an illustration, Moderna, which became famous for its COVID-19 vaccine, is now working hard on advancing its mRNA technology in oncology. 'Our personalized approach, particularly our progress in treating melanoma, has shown promising results, with significant improvements in survival rates. These therapies, developed in collaboration with Merck, leverage the immune system's capabilities to better recognize and combat cancer cells, illustrating the potential of mRNA technology in oncology,' Moderna's CEO, Stéphane Bancel shared.
But what transpired from our numerous interviews is that the new superstars in oncology are cell and gene therapies. More than 1,000 such programs are currently in development and a good deal of them are meant to address hard-to-treat cancers. As Thermo Fisher Scientific's president of Biosciences, Amy Butler, put it: 'Cell and gene therapies hold tremendous potential to be curative for diseases that were previously deemed untreatable, such as childhood leukemia and breast cancer. These therapies offer a revolutionary approach by potentially eliminating the disease rather than merely managing symptoms.' Smaller biotechs tend to be the main drivers of innovation in this area, but pharmaceutical giants have a pivotal role to play too, especially due to their ability to channel considerable resources. Sebastian Guth, the COO of Bayer Pharmaceuticals and president of Bayer US, corroborated: 'Bayer has actively participated in this evolution by investing €3.5 billion in building our cell and gene therapy platform. We have made strategic acquisitions and partnerships, such as Ask Bio for gene therapies and Blue Rock Therapeutics for cell therapies, to strengthen our position.'
Perhaps the most popular amongst cell and gene therapies are chimeric antigen receptor (CAR) T-cell therapies. One can consider CAR Ts as the vanguard in oncology, particularly since the FDA has approved only six of them to date (the first approval was granted in 2017). They are a type of immunotherapy that involves the administration of genetically modified T-cells (the most powerful component of the immune system), which allow for greater precision in targeting cancer cells. Recent breakthroughs in this space indicate that these therapies may be a game-changer in oncology.
CAR Ts have demonstrated considerable success in treating deadly blood cancers, often leading to long-term remissions. All six FDAapproved CAR T-cell therapies, developed by Novartis, Kite Pharma, BMS and Janssen, target liquid tumors (blood and plasma). The efficacy of these treatments can vary and certain issues, such as cancer resistance and toxicity, persist. We spoke with a clinical-stage biotech, CARGO Therapeutics, that tries to overcome cancer resistance and increase the efficiency of developed CAR T-cell programs. 'Current CD 19-targeted CAR T-cell therapies have set a transformative precedent in large B-cell lymphoma treatment, with about 40 percent of patients achieving long-term complete response,' the company's CEO, Gina Chapman told us. Their goal at CARGO, however, is to address the 60 percent of patients for whom these therapies are not effective. 'Preliminary results are promising, showing a 53 percent complete response rate in this subgroup, which is remarkable considering these are patients with very limited options and a median survival of less than six months prior to treatment,' Chapman added. CARGO's lead candidate, firi-cel (CRG-022), is currently in Phase 2 trials.
An unintended consequence of innovation in health care is the widening gap between those who can access complex and costly therapies, and those who cannot. True innovation must prioritize broad access to care and this is a major focus for LLS. E. Anders Kolb, M.D., President & CEO, The Leukemia & Lymphoma Society (LLS)
CAR T-cell therapies can be autologous and allogeneic. In the case of autologous (from Ancient Greek autós, 'self'), T-cells are taken from the patient being treated, they are genetically modified and reinserted into their body. The aim is for the boosted T-cells to then destroy the cancer. The other type, allogeneic (from állos, meaning 'other'), involves a similar process, but cells are in that case provided by a healthy donor for multiple patients. Autologous treatments are the only ones currently available on the U.S. market. But more and more companies are turning toward the allogeneic alternative, which has notable advantages in its scaling potential. 'Allogeneic CAR T products are developed using T-cells from healthy donors. These cells are isolated in a manufacturing facility, engineered to express CARs to recognize and destroy disease, and modified via gene editing to limit autoimmune response when given to a patient,’ explained Dr. Zachary Roberts, EVP of R&D and chief Medical Officer of Allogene Therapeutics. The company, whose lead program targeting B-cell malignancies is currently in Phase 2, claims to have overcome the issue of autoimmune reactions by additional gene editing. 'Our products are produced in advance, stored, and ready for rapid administration, drastically reducing the time from patient eligibility to treatment commencement,' Roberts added.
While we are still to see the first FDA green light in this space, the European Commission granted the first approval globally for an allogeneic T-cell immunotherapy back in 2022. The therapy, called Tabelecleucel and targeting relapsed/refractory post-transplant lymphoproliferative disorder (PTLD), was developed by California-based Atara Biotherapeutics. 'Our T-cell therapy has shown around 50 percent response rate in treating relapsed/refractory PTLD, leading to long-term survival in responders. This represents significant progress in a deadly disease with no approved therapies,' Atara's president & CEO, Pascal Touchon, added.
But while CAR T-cell treatments have made considerable strides in treating certain blood and plasma malignancies, they have fallen short in targeting solid tumors. This has meant that more traditional approaches like chemotherapies and surgeries continue to be the primary options for solid tumors, which are also 90 percent of all cancers. The great news is that another type of T-cell therapy may be able to cut this Gordian knot. It was this January that the FDA approved the first cellular therapy for a solid tumor—the drug, called Amtagvi (lifileucel), is for metastatic melanoma and was developed by California-based Iovance Biotherapeutics. 'This achievement opens a new frontier in oncology,' said Fred Vogt, Iovance's interim CEO. In addition to melanoma, Iovance's treatment has already shown promising results for non-small cell lung cancer.
But cell therapy and concretely CAR Ts hold promises that go beyond oncology. 'One remarkable instance involves a patient who suffered from Myasthenia gravis for years and became immobilized in a wheelchair. She received a single dose. Within months, she regained the ability to walk—and she even surpassed her own husband's stamina on a hike recently,' shared Peter Maag, the CEO of Kyverna Therapeutics. Maag is confident that his company is on the verge of revolutionizing the treatment of autoimmune diseases. 'At a high level, autoimmune diseases involve the immune system attacking itself. We extract T cells from the patient, genetically re-engineer them, and re-inject them. It reminds me of resetting a computer to get everything back to normal,' Maag said. Kyverna's lead candidate, KYV-101, is already in Phase 2 for certain indications. Maag told us that part of its therapeutic allure stems from its prospective to offer a long-term remission for patients. Kyverna is not alone, as other biotechs, like Atara, also perceive the potential of cell therapies to fight autoimmunity.
Innovations in immunotherapy are not restricted to cell and gene therapies. Xilio Therapeutics, for example, is developing tumor-activated immunotherapies that allow for greater precision and, thereby, far less toxicity for healthy tissues. Systemic toxicity has been a principal problem of modern immunotherapies. Oftentimes patients' tumors are successfully shrinking, but the therapy has to be discontinued due to overwhelming side effects. Xilio's president & CEO, Dr. René Russo, shared: 'We have discovered that we can activate our first molecule, an anti-CTLA-4 monoclonal antibody, predominantly within the tumor—showing 70 to 90 percent activity in the tumor environment, while maintaining less than 15 percent activity in the circulating blood.
Our geographic precision medicines are tumor-selective immunotherapies designed to focus the immune system's tumor-destroying effect locally in tumor tissue but not in healthy tissues. By localizing activity to the tumor, we hope to overcome the toxicities of prior generations of immunotherapies. René Russo, CEO, Xilio Therapeutics
This targeted activity allows us to minimize side effects significantly, thereby enabling higher dosages and longer treatment durations.' Xilio is also developing two cytokine programs with the same objective, as cytokines have been known for their side effects. 'We have been able to administer significantly higher doses than previously possible, thanks to the tumor-selective activation of these molecules,' Russo told us, adding that these can now work in conjunction with cell therapies too.
Another company bent on solving the challenge of cytokine-based therapies is Werewolf Therapeutics. 'Werewolf' is a metaphor for the company's objective: 'They are designed to be delivered systemically and remain inactive throughout the patient's body, akin to a werewolf in daylight. However, upon entering the tumor microenvironment—comparable to the moonlight for a werewolf—our drugs are designed to transform into aggressive agents to stimulate a powerful immune response and unleash an attack on cancer cells,' Dan Hicklin, Founder & CEO of the biotech explained. 'Our solution involves creating cytokine prodrugs, called INDUKINE molecules, that remain inactive in circulation but activate upon reaching tumor tissue', Hicklin added. Werewolf is focused on treatments for melanoma, lung and kidney cancers.
After the many interviews we had in the oncology space, we concluded that thinking of cancer as a single disease is outdated and erroneous. Cancer has many forms and each of these may present differently in different patients. This is the great attraction of personalized medicine in oncology. That also means that, if we are to be successful in solving cancer(s), many different solutions will be needed—cell and gene therapies, radiopharmaceuticals, mRNA, cytokines… The list, most certainly, is yet to grow. And that is good news.
Uncovering cancer
Tackling deadly diseases like cancer via advanced therapeutics is only one side of the coin; the other is early detection. Luckily, breakthroughs in diagnostics are also proliferating.
Every health system and, naturally, every individual would be greatly relieved if a universal early detection cancer test were discovered. We spoke with a company that claims to have made significant progress in that direction. GRAIL has developed a multi-cancer, early detection blood test called Galleri that 'can identify over 50 types of cancers, focusing particularly on highly lethal cancers that lack existing screening modalities in population health, such as pancreatic, esophageal, and liver cancers,' GRAIL's CMO, Jeffrey Venstrom told us, emphasizing that nearly 70 percent of cancer deaths are caused by cancers without recommended screening. The Galleri test identifies genomic features and abnormal DNA fragments in one's blood, employing advanced techniques to also provide information about a tumor's location. But, with about 40 percent of positive predictive value for the moment, the test is still not to be used in isolation and at the expense of traditional screening. 'Over the next few years, our primary goal is to secure broad adoption and FDA approval through our ongoing clinical trials and our breakthrough designation status,' Venstrom added. GRAIL has also initiated a collaboration with CMS (Centers for Medicare and Medicaid Services) to conduct a real-world implementation study, called the REACH, targeting the Medicare population.
Every year in the U.S., over 2M patients with cancer are at risk of infections. Karius aims to significantly reduce the rate of deaths and complications in patients with cancer who are among the most vulnerable to infections. Providing the latest scientific advancements in the detection of pathogens to improve patient outcomes, is a core value of our work. Alec Ford, CEO, Karius
Others, like Personalis, are targeting patients who have already had cancer. 'Our focus is on individuals with a high risk of recurrence, where the value and impact of early detection justify the cost. As technology costs decrease, expanding to broader early detection becomes more feasible,' reasoned Christopher Hall, the CEO of Personalis. The Bay Area biotech's technology is centered on the identification of Minimal Residual Disease (MRD) and utilizes whole-genome analysis to create a unique tumor profile for each patient.
As we find out, however, treating patients with cancer is not just about cancer. 'Over half of all cancer deaths are due to infections, not the cancer itself,' said Alec Ford, the CEO of Karius—a company that provides rapid and accurate diagnosis of infections, notably focusing on oncology patients. Cancer patients are exceptionally vulnerable to infections, Ford explains, adding that traditional approaches to infectious diagnosis are slow and cumbersome, threatening many of these patients' lives. 'By analyzing a small blood sample, within just 24 hours we can identify microbial DNA from over 1,000 pathogens, including bacteria, fungi, parasites, and viruses,' Ford highlighted the benefits of the Karius test. At any rate, the examples of GRAIL, Personalis and Karius demonstrate that, in oncology-related diagnosis too, multiple lines of action are required.
The hidden catalysts of medical innovation
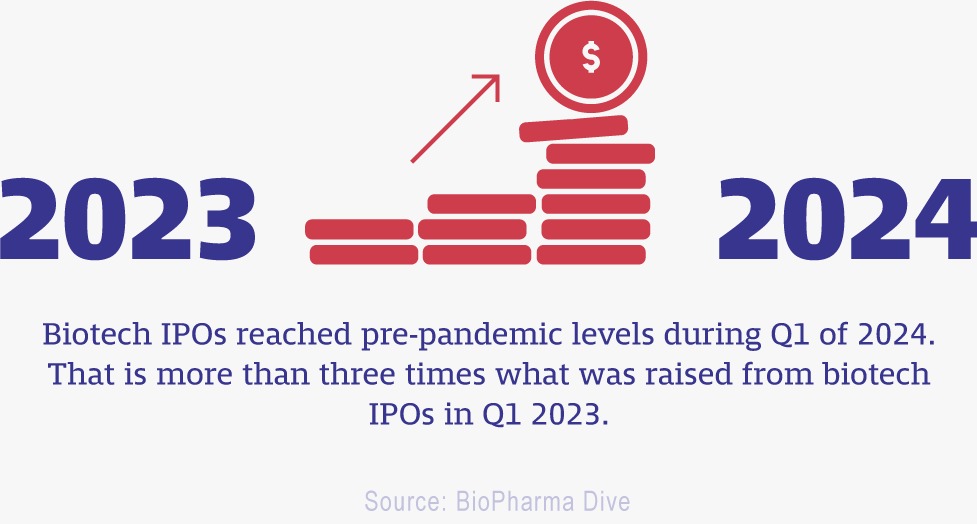
Beyond scientific conundra, a major concern associated with most of these novel therapies is their accessibility. This is an issue inherent to personalized medicine. While much more precise, potent and sparing for the patient, it involves significantly more resources. As in the case of autologous cell therapies, an entire team of scientists may be engaged to work on the modification of the cells of a single patient. In fact, a record was recently broken with the price of Orchard Therapeutics' (a subsidiary of Kyowa Kirin) Lenmeldy gene therapy drug set at $4.25 million. 'The pricing really reflects the profound impact hematopoietic stem cell gene therapies can have—helping transform a fatal, devastating disease into something that may be addressed with a one-time treatment. It also recognizes the significant investment required to bring such innovative, personalized treatments to market for an ultra-rare disease,' Kyowa Kirin North America's president, Steve Schaefer explained. Whereas these are certainly valid points, the question of accessibility remains to be solved.
As mentioned, specifically in the CAR T-cells space, costs can be tempered via the allogeneic card, which allows for the production of multiple doses from a single manufacturing run. Yet, solutions do exist for autologous therapies, which can have important advantages in certain cases. Service providers, like Contract Development and Manufacturing Organizations (CDMOs), can have a key role to play here. Cellares' CEO & Co-Founder, Fabian Gerlinghaus, emphasized the degree of the scalability problem: 'About 20 percent of patients are dying on the waitlist even though they are eligible for approved cell therapies because the industry is unable to meet patient demand.' Cellares has developed a manufacturing platform, called Cell Shuttle, which, Gerlinghaus claims, successfully integrates and automates the entire cell therapy manufacturing process into one place. 'It encapsulates the functionality of approximately 100 benchtop instruments in a single, compact machine,' Gerlinghaus said, adding that this 'reduces labor and space requirements by 90 percent. The other key difference is that the Cell Shuttle can process 16 cell therapy processes simultaneously.' Gerlinghaus anticipates that the Cell Shuttle will be able to meet worldwide demand for cell therapies in the future.
Cellares is an example of how contract service providers can play a critical role in supporting the work of biopharma companies. Another such illustration comes from Nucleus RadioPharma, a CDMO operating in the space of radiopharmaceuticals. Highly promising, such therapies rely on low-energy isotopes that integrate cancer-targeting molecules. Radiopharmaceuticals can allow for precise tumor treatment while minimizing damage to surrounding tissues. 'The potential in this field is significant, especially for conditions like neuroendocrine tumors and prostate cancer, where targeted radiotherapy could have a transformative impact,' Théodore Leondaridis, the Global Oncology Head of Pierre Fabre Group shared with us. The company is considering its entry into this space, as the sector is attracting more and more attention, as exemplified by recent news about AstraZeneca's pending $2.4 billion acquisition of Fusion Pharmaceuticals, known for developing next generation radioconjugates. But for all their prospects, radiopharmaceuticals have an Achilles' heel—manufacturing and logistics. Nucleus RadioPharma's CEO, Charles Conroy, told us that the industry is particularly challenging 'due to the short half-life of isotopes, which complicates logistics similar to shipping ice without refrigeration.' To address this, the CDMO is establishing multiple production sites across the U.S. 'We are strategically situating our facilities near major medical centers to expedite the transfer of isotopes and drugs into patient studies and eventual commercial use,' Conroy said. But while infrastructural issues persist, Conroy is optimistic that the large investments that the sector is attracting will solve these. The increased relevance of contract service providers is a general trend in life sciences driven as much by economic considerations, as by new challenges that come along with scientific advancements. 'The shift from small molecules to large molecules and biologics marks a significant evolution in health care. This transition necessitates specialized expertise in areas like cell and gene therapy, where CDMOs excel,' observed William Humphries, the CEO of Alcami Corporation. To respond to this new reality, global service providers are expanding and/or profiling their activities. 'We have broadened our service scope by acquiring CELLforCURE from Novartis, a state-of-the-art cell and gene therapy manufacturing unit. This acquisition allows us to offer a more comprehensive range of CDMO activities, extending our expertise from small molecules to cell and gene therapy,' the CEO of the French-based SEQENS, Pierre Luzeau, told us.
Crucially, more than meeting new demands, service providers are also proactive innovators. A telling illustration comes from Codexis, which offers engineered enzymes to its various clients. One of its platforms, ECO Synthesis, is designed to address the scalability and sustainability challenges that have characterized the RNA synthesis space. Particularly the latter is a topic that has received little media attention but, as we are told, conventional RNA synthesis is heavily reliant on environmentally detrimental solvents. 'ECO Synthesis operates in water, dramatically reducing the carbon footprint and eliminating the need for large-scale, expensive containment facilities. This innovation not only promises a more sustainable approach but also significantly reduces capital investment requirements, enabling the production of siRNA at scales previously deemed unfeasible,' the CEO of Codexis, Stephen Dilly noted. Innovations of this sort are likely to prove to be as the key differentiator in the space of contract service providers in the years to come.

